For more on this story, listen to James Tapper’s episode of the Sensemaker, A breakthrough in treating Huntington’s disease, here.
Anna Sheahan turned 21 last week – her first birthday since her mother, Charlotte Raven, died from Huntington’s disease (HD).
The illness cast a long shadow over their family. Raven had been one of British journalism’s spikiest voices, as an editor of the Modern Review and a writer for other publications, including The Observer, but HD gave her wild mood swings and jittering hands that could not type properly. It dulled her mind and, eventually, in January, killed her. The disease came with the added torment that it is both hereditary and incurable.
Then, last Wednesday, Sheahan received a text from Prof Edward Wild, a consultant neurologist who had co-written a book with her mother, Patient 1, about an experimental treatment that ultimately had not worked. “Check the one o’clock news,” the message read.
‘The news came on my 21st birthday. It was crazy. He said: ‘That’s the way the universe works sometimes, isn’t it?’
‘The news came on my 21st birthday. It was crazy. He said: ‘That’s the way the universe works sometimes, isn’t it?’
Anna Sheahan, daughter
The student switched on her TV and watched as Wild and his colleague at University College London’s (UCL) Huntington’s Disease Centre, Prof Sarah Tabrizi, announced that HD was no longer untreatable. They had managed to slow the disease’s progression by as much as 75%. It meant someone receiving the treatment may never suffer the symptoms of Huntington’s. It was spectacular.
“I said to him: ‘No way, Ed, you’ve just announced this on my birthday,’” Sheahan said. “He said: ‘That’s the way the universe works, isn’t it?’ It was crazy. I watched the news, had a bottle of prosecco, then I went to the pub.”
For Wild, the announcement was the fulfilment of a promise. “After setback after setback after setback, it’s immensely gratifying to discover, after 20 years, that you’re not on the wrong track,” he said.
“A lot of my closest friends [are affected by HD] and the idea of not disappointing [them] has a lot going for it. Huntington’s is a neurodegenerative disease caused by a genetic mutation, a corruption of DNA in the huntingtin gene, where three letters of the genetic code – C, A and G – are repeated. The more repeats, the more likely it is to cause the disease by asking RNA messengers to produce a protein that damages brain cells.
What researchers have been working on is finding a way to get a drug to bind to and cancel the messengers – known as RNA interference. The new drug – AMT-130 – created by uniQure, a Dutch company, and tested by UCL and other researchers, aims to do exactly that.

“[The drug is] basically telling the cell to delete the RNA and the protein doesn’t get made,” Wild said.
Newsletters
Choose the newsletters you want to receive
View more
For information about how The Observer protects your data, read our Privacy Policy
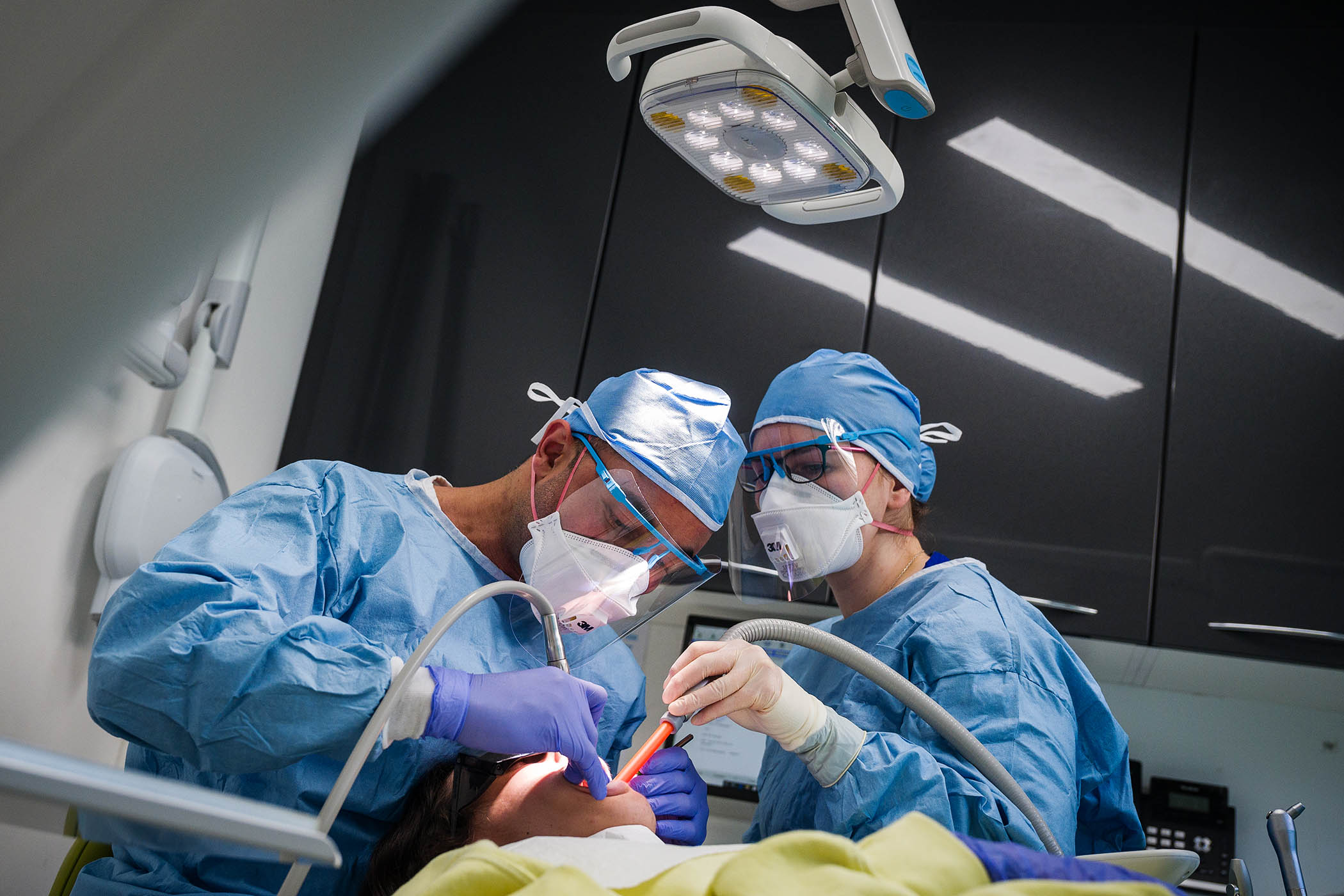
But how do you deliver such a drug? “Very, very carefully,” said Prof William Gray of the Advanced Neurotherapies Centre at Cardiff University, one of the few places in the world and the only one in the UK with the capability of performing gene therapy.
AMT-130 has to be delivered directly into the striatum, one of the deepest parts of the brain, which we use to move and to control our moods, and is shrunk and damaged by Huntington’s.
Gray and his team drilled six holes into the skulls of each of their four patients, then fed catheters 12cm deep into the brain to deliver a few hundred microlitres of AMT-130.
The precision required meant the entire operation was done inside an MRI scanning unit, so the surgeons could check where their surgical instruments and catheters were going at every step of the operation. Some took 18 hours.
“The patients got a few hundred MRI scans to do this procedure,” Gray said.
It took two years to set up the trial and each surgery needed two months of preparation. “These are very, very brave people. This stuff isn’t without risk. They’re entering the unknown and may get no benefit,” Gray added.
In a call to investors, uniQure estimated the cost would be similar to other gene therapies, which can be more than $2m a patient. Wild thinks this would be justifiable.
“Gene therapies are expensive, but treating people’s symptoms and looking after people with HD is very expensive,” Wild said. Delaying the disease in an 18-year-old so symptoms arrive when they are 108 rather than 40 justifies the expense, he added.
Even so, it would take years for Gray’s team to perform surgery on the 8,000 people in the UK who need treatment and many others among their families who may carry the huntingtin gene but have not been tested.
Sheahan had decided never to get tested “unless I was thinking of having children or getting married”, she said. “But this changes everything.”
Dr Lauren Byrne works with Wild at UCL. Her father died of HD in February. She has been tested and was negative, but not everyone in her extended family agrees. “The last time I counted, it’s approaching about 40 people at risk [in my family],” she said.
“My family is pretty positive and gets on with things. But there definitely was a sense of ‘don’t get tested’. My cousin [died by] suicide two days after finding out he had the gene back in the 90s.”
Byrne researches HD biomarkers – biological signs that show how far the disease has progressed. This is almost as vital as the cure itself.

.
It would be unethical to withhold treatment from people and wait for their disease to get worse, she said, and HD develops so slowly that it can be hard to tell whether an intervention is working.
“I get a lot of mixed emotions,” Byrne said. “It’s an amazing result. But I feel responsibility to my family and all the HD families. People are desperate for this hope. But we still have a road to follow.”
Hopes have been dashed before. Raven was convinced that the trial she took part in would save her life. “When she was on the trial, that was the best year she had when she was ill,” her daughter said. “She kind of came alive again.”
The day her mother was told the trial was stopping was devastating. “It set her in a bad way for a long time,” said Sheahan.
When the euphoria subsides, Wild and other researchers will be back in the lab working on ways to make AMT-130 and other treatments more easily available.
Trials of spinal injections or even a tablet are already under way. Gray and his colleagues are also working on ways to make the surgery quicker.
“I just wish I could have given Charlotte an idea that this was about to happen for [her children] if they need it, and for everyone else,” Wild said. Even the medicine that Raven helped test may yet become available. It is being investigated at a phase II trial to establish whether a lower dose may be more effective without side-effects.
“This would have been such a massive deal for her,” Sheahan said. “Regardless of if it could help her or not. She saw her dad die from it. I saw her die from it. And she would know that no one else is going to.”
Portrait by Katherine Anne Rose for The Observer. Charlotte and Anna photographed by Linda Nylind. Other picture courtesy of UCL